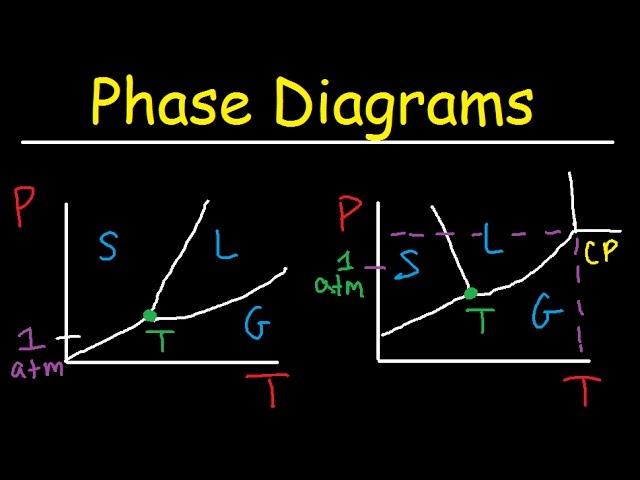
A water phase diagram is a graphical representation of the physical states of water (solid, liquid, and gas) as a function of temperature and pressure. This diagram is crucial for understanding water’s behavior under various conditions, from the freezing of ice to the boiling of water, and even the existence of exotic phases like supercritical water. Its applications range from meteorology and climatology to chemical engineering and materials science. Analyzing this diagram provides insights into the transitions between different phases and helps predict the state of water under specific environmental pressures and temperatures. The diagram’s simplicity belies the complex interplay of intermolecular forces that govern these phase transitions.
The water phase diagram illustrates the three primary phases of water: solid (ice), liquid (water), and gas (water vapor). Each region on the diagram corresponds to a single phase, while the lines separating these regions represent phase transitions. The critical point, where the distinction between liquid and gas vanishes, is also prominently featured. Understanding the phase diagram allows for predictions regarding the behavior of water under diverse conditions, which is vital in numerous scientific and engineering applications. This diagram provides a fundamental framework for comprehending waters thermodynamic properties.
The triple point, where all three phases coexist in equilibrium, is another significant feature of the water phase diagram. It signifies a unique set of temperature and pressure conditions where all three states of matter can simultaneously exist. This point is of immense importance for calibration in thermometry and other precision measurements. Furthermore, the slope of the solid-liquid equilibrium line is unusual, showcasing water’s anomalous behavior with its density decreasing upon freezing. This property has far-reaching consequences for various natural processes and technological applications.
Understanding the Water Phase Diagram
The diagrams axes represent temperature and pressure, allowing for a straightforward visualization of water’s phase transitions. The curves separating the phases represent points of equilibrium between two phases (e.g., the melting curve shows conditions where ice and water coexist). The pressure axis generally ranges from low vacuum to high pressure, while the temperature axis covers a range relevant to the phases of water typically encountered. Detailed examination of the diagram reveals the subtle yet crucial interrelationship between temperature, pressure, and the physical state of water. Knowledge of the water phase diagram allows precise predictions on the behavior of water in various situations and environments.
Careful observation of the water phase diagram reveals several key features, including the triple point, critical point, and the negative slope of the solid-liquid equilibrium line. These features highlight the unique thermodynamic properties of water, differentiating it from many other substances. Its important to remember that this diagram is a simplified model, and deviations can occur due to impurities or other external factors. Despite these slight variations, the diagram remains a crucial tool for understanding waters behavior across a wide spectrum of conditions.
-
Define the Axes:
Establish a temperature axis (typically in Celsius or Kelvin) and a pressure axis (usually in atmospheres or Pascals). The temperature range should encompass the melting and boiling points of water, and the pressure range should include relevant atmospheric and higher-pressure conditions. Accurate scaling is essential for proper representation of the phase boundaries.
-
Plot the Phase Boundaries:
Using experimentally determined data or established values, plot the curves that separate the solid, liquid, and gas phases. These curves represent the conditions under which two phases are in equilibrium. Accurate plotting requires precise data points and careful consideration of the scale chosen for the axes. Pay close attention to the peculiar negative slope of the ice-water boundary.
-
Mark Key Points:
Clearly mark the triple point (where all three phases coexist) and the critical point (where the distinction between liquid and gas disappears). These points represent significant thresholds in the water phase diagram and hold particular importance in various scientific and engineering applications. Correct identification and labeling of these points are critical for the overall clarity and accuracy of the diagram.
Frequently Asked Questions about the Water Phase Diagram
The water phase diagram, while seemingly simple, often raises questions regarding its interpretations and applications. This section will address some commonly asked questions to clarify any ambiguities and provide a deeper understanding of this essential tool in understanding the physical properties of water. Understanding the intricacies of the diagram can lead to more effective problem-solving in various fields.
What is the significance of the negative slope of the solid-liquid equilibrium line?
The negative slope is unique to water and arises from the anomalous density behavior of ice. Unlike most substances, ice is less dense than liquid water, meaning the solid phase occupies a larger volume. This has significant implications for aquatic life, as it prevents bodies of water from freezing solid from the bottom up. This unusual property also affects various geological processes and industrial applications involving water.
What is the critical point and its importance?
The critical point marks the temperature and pressure above which the distinction between liquid and gas phases disappears. Beyond the critical point, water exists in a supercritical fluid state, which possesses unique properties that are utilized in various industrial processes, such as extraction and chemical reactions. This point represents a fundamental transition in the behavior of water.
How is the water phase diagram used in meteorology?
Meteorologists use the diagram to understand cloud formation, precipitation, and other atmospheric phenomena. By analyzing temperature and pressure data, they can predict whether water will exist as vapor, liquid droplets, or ice crystals in the atmosphere, contributing to accurate weather forecasting and understanding climatic patterns. It forms the basis for interpreting weather patterns.
The water phase diagram provides a valuable tool for understanding various natural processes and industrial applications. Its use extends far beyond simple visualization, allowing for precise prediction of water behavior under diverse circumstances. The unique properties of water highlighted by this diagram are crucial for comprehending many important phenomena in various fields. Further study may reveal more nuances of this essential diagram.
The simplicity of the diagram can be deceptive, hiding the complexity of intermolecular forces at play in different phases. It is essential to understand the interplay between pressure and temperature to accurately predict and interpret the behavior of water based on the phase diagram. Therefore, a robust understanding of thermodynamics is needed for full comprehension.
It’s crucial to emphasize that the water phase diagram is a model, and real-world conditions can show some variations due to factors like the presence of impurities or external forces. However, it offers an excellent approximation, crucial for numerous scientific and engineering applications.
Key Aspects of the Water Phase Diagram
The water phase diagram, as a noun, represents a key concept in understanding the physical properties of water. Its importance stems from its ability to visually depict the states of matter under varied conditions. The diagram’s usefulness spans various fields. Studying its components and implications enhances comprehension of water’s behavior under varied circumstances.
Temperature Dependence
Temperature is a crucial factor determining the phase of water. Increasing temperature generally leads to phase transitions from solid to liquid to gas. This relationship is clearly demonstrated on the diagram. The transition points are precisely defined by the phase boundaries, providing a clear understanding of temperature’s role in phase transitions.
Pressure Dependence
Pressure significantly influences the boiling and freezing points of water. Higher pressure raises the boiling point and lowers the freezing point, as seen in the diagram’s slopes. This is crucial in high-altitude cooking, where water boils at lower temperatures, and in deep-sea environments where immense pressure impacts phase transitions. Understanding the pressure effect on phase transitions is crucial for many applications.
Phase Transitions
The lines on the diagram represent the conditions where two phases are in equilibrium (e.g., melting, boiling, sublimation). These transitions are characterized by changes in enthalpy and entropy. The precise points of transitions are vital in various scientific and engineering processes requiring precise control of water’s state. Understanding these phase transitions is crucial for various industrial and environmental applications.
Triple Point
The unique point where solid, liquid, and gas phases coexist in equilibrium holds significant importance for calibration in thermometry and precision measurements. This point represents a well-defined state used as a reference point for temperature scales. Knowing the characteristics and significance of this point is crucial in scientific measurement practices.
Critical Point
The critical point, beyond which the liquid-gas distinction disappears, opens up the possibility of utilizing supercritical water, which possesses remarkable solvent properties. This knowledge finds applications in various industrial settings and research fields. The understanding of supercritical fluids has greatly expanded the applications in chemistry and chemical engineering.
The interrelationship of temperature, pressure, and the resulting phases is clearly visible in the diagram. The diagram’s simplicity allows for easy visualization of complex thermodynamic behaviors. The ability to predict water’s behavior under various conditions is a powerful application of the diagram.
In conclusion, understanding the water phase diagram is paramount for anyone working with water across various scientific and engineering disciplines. Its detailed visualization provides insight into the complex relationships between temperature, pressure, and phase behavior.
Tips for Understanding and Using the Water Phase Diagram
Mastering the water phase diagram requires a systematic approach that combines visual understanding with theoretical knowledge of thermodynamics. By focusing on key points and features, one can effectively utilize this tool for a range of applications.
Practice interpreting the diagram under varying conditions. Start by identifying the state of water given specific temperatures and pressures and vice versa. This will enhance understanding and improve predictive capabilities.
Start with the Basics
Begin by familiarizing yourself with the axes of the diagram, the regions representing different phases, and the key points such as the triple point and the critical point. Understanding the basic components is essential before moving towards advanced concepts.
Analyze Phase Transitions
Focus on understanding the phase transition lines and what they represent the conditions where two phases are in equilibrium. Understanding how changes in temperature and pressure influence these transitions is crucial.
Practice Problem Solving
Solve practice problems that involve determining the phase of water given specific temperature and pressure values or vice-versa. This hands-on application helps in solidifying the theoretical knowledge gained from studying the diagram.
Explore Advanced Concepts
After mastering the fundamentals, move on to explore advanced concepts such as the concept of supercritical fluids and the implications of the unique properties of water, as depicted in the diagram.
Relate to Real-World Examples
Relate the concepts learned from studying the phase diagram to real-world examples. Consider how the diagram applies to everyday observations such as boiling water, ice formation, or weather patterns. This aids in developing a holistic understanding of its significance.
The water phase diagram serves as a foundational tool for numerous applications, ranging from predicting weather patterns to optimizing industrial processes. It is crucial to remember that the diagram is a simplification of a complex system and that variations may occur in real-world scenarios. However, this simplified representation remains extremely powerful.
Understanding its intricacies requires careful study and practice. Through a systematic approach, one can effectively utilize this tool and gain deep insights into the thermodynamic behavior of water.
In conclusion, the water phase diagram is a vital tool for understanding and predicting the behavior of water under various conditions. Its applications extend across numerous scientific and engineering disciplines, highlighting its importance as a fundamental concept in thermodynamics and material science. The diagram is a simple yet powerful representation of the complexity of water’s physical behavior.
Youtube Video: