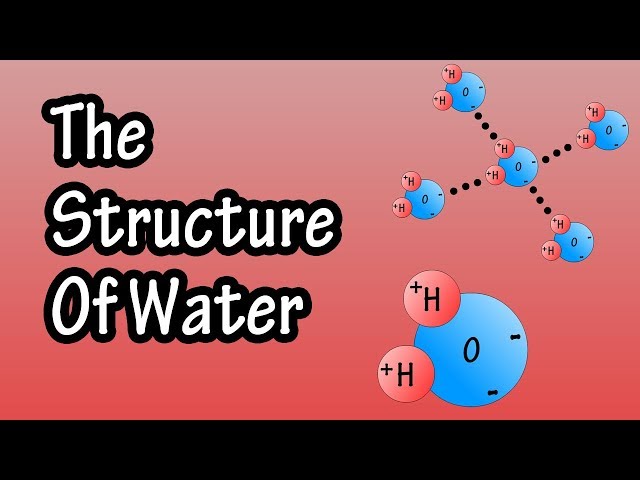
A water molecular diagram is a visual representation of the structure of a water molecule, showing the arrangement of its atoms and bonds. Understanding this diagram is fundamental to comprehending many of water’s unique properties, from its high boiling point to its ability to act as a solvent. These diagrams are crucial in various scientific disciplines, including chemistry, biology, and environmental science. The simplicity of the visual aids understanding of complex chemical interactions. Accurate depictions are essential for effective communication and education.
The water molecular diagram illustrates the covalent bonds between two hydrogen atoms and one oxygen atom. Oxygen, being more electronegative, attracts the shared electrons more strongly, creating a slightly negative charge near the oxygen and slightly positive charges near the hydrogens. This polarity is key to water’s ability to form hydrogen bonds with other water molecules and other polar substances. A thorough understanding of the water molecular diagram allows for a deeper appreciation of the molecule’s behavior and its significance in various contexts. The diagram’s simplicity belies the complexity of the interactions it represents.
Water’s unique properties, such as its high surface tension and specific heat capacity, are directly related to the structure depicted in the water molecular diagram. This structure also explains water’s behavior as a universal solvent, dissolving many ionic and polar compounds.
Understanding the Water Molecular Diagram
The arrangement of atoms in a water molecular diagram is crucial for understanding its properties. The bent molecular geometry, with a bond angle of approximately 104.5 degrees, is a consequence of the lone pairs of electrons on the oxygen atom. This bent shape influences the molecule’s polarity and interactions. The diagram simplifies a complex three-dimensional structure into a two-dimensional representation for easier comprehension. Analyzing different representations of the water molecular diagram, including ball-and-stick and space-filling models, provides further insights into its structure.
Accurate representation of the water molecular diagram is essential for effective communication of scientific concepts. Different diagram types, like those showing electron distribution or hydrogen bonding, enhance understanding of specific properties. These diagrams are commonly used in educational settings and scientific publications for clarity and understanding. Moreover, the use of such diagrams is not limited to academic fields but expands into various industries such as water purification and wastewater treatment.
-
Step 1: Draw the Oxygen Atom.
Begin by drawing the central oxygen atom (O). This atom is larger and more electronegative than the hydrogen atoms. Remember that oxygen has six valence electrons; two are involved in bonding with the hydrogen atoms and four remain as two lone pairs. These lone pairs are crucial in determining the molecular geometry. Accurate representation of the atom’s size is important for a clear visualization.
-
Step 2: Add the Hydrogen Atoms.
Next, add the two hydrogen atoms (H) to the oxygen atom. Each hydrogen atom shares one electron with the oxygen atom to form a covalent bond. The hydrogen atoms are placed at an angle, not linearly, to depict the bent molecular geometry. The bond angle should be approximately 104.5 degrees for accuracy.
-
Step 3: Show the Bonds.
Represent the covalent bonds between oxygen and hydrogen using lines. Each line represents a shared pair of electrons. This step visualizes the electron sharing aspect of the covalent bonds, a key characteristic of the molecule. This is often shown as a single line for single bonds.
-
Step 4: Indicate Partial Charges (Optional).
For a more advanced diagram, you can indicate the partial charges (+ and -) on the hydrogen and oxygen atoms, respectively. This emphasizes the polarity of the water molecule which is crucial in its interactions with other molecules and its properties. This visual cue aids understanding of the polar nature of the water molecule.
Frequently Asked Questions about Water Molecular Diagrams
Water molecular diagrams are frequently used in educational and scientific contexts to illustrate the structure and properties of water molecules. Many questions arise regarding their creation, interpretation, and applications. Understanding the answers helps in gaining a comprehensive understanding of this important molecule and its behaviour. The accurate depiction of the water molecular diagram is important for understanding its role in various processes. Addressing common misconceptions through FAQ improves knowledge on the topic.
What is the significance of the bond angle in a water molecular diagram?
The bond angle of approximately 104.5 degrees in a water molecular diagram is a critical feature. Its not 180 degrees (linear) due to the presence of two lone pairs of electrons on the oxygen atom. These lone pairs repel the bonding pairs, pushing the hydrogen atoms closer together. This bent shape leads to the molecule’s overall polarity and significantly influences its properties, including its ability to form hydrogen bonds.
How does a water molecular diagram help explain water’s unique properties?
The water molecular diagram directly relates to water’s unique properties. The bent shape and polarity explained by the diagram are responsible for hydrogen bonding. These bonds are stronger than many other intermolecular forces, resulting in water’s high boiling point, high surface tension, and its exceptional ability to act as a solvent for many ionic and polar compounds. These properties are vital in many biological and environmental processes.
Are there different types of water molecular diagrams?
Yes, several types of water molecular diagrams exist, each highlighting different aspects of the molecule’s structure. These include simple Lewis structures, ball-and-stick models, space-filling models, and diagrams showing electron density and partial charges. The choice of diagram depends on the specific information being conveyed. Each visualization method conveys the structure and properties of water in a unique way that caters to a specific need or perspective.
The water molecular diagram is a fundamental tool for understanding water’s unique properties. Its simplicity allows for clear communication of complex concepts. The diagram illustrates the covalent bonds and molecular geometry that give rise to polarity and hydrogen bonding. Accurately depicting the water molecular diagram is paramount for conveying the key characteristics of this essential substance.
The diagram’s representation of the molecule’s structure is key to grasping the importance of water’s role in many aspects of life and chemistry. It helps to understand how water functions as a solvent, contributes to various chemical processes, and impacts various environmental phenomena. A solid understanding, aided by the visual representation of the water molecular diagram, is crucial to comprehending the significance of water in the world around us.
Key Aspects of a Water Molecular Diagram
Analyzing a water molecular diagram reveals key structural and behavioral aspects of the molecule. These aspects explain various properties and interactions. The visual representation facilitates understanding of complex scientific concepts. The diagram serves as a cornerstone for learning and further investigation.
Covalent Bonds
Covalent bonds in a water molecular diagram represent the shared electron pairs between oxygen and hydrogen atoms. These bonds hold the molecule together and are essential for its stability. Understanding covalent bond formation and electron sharing is key to comprehending the molecule’s properties. The strength of these bonds dictates the stability of the molecule under various conditions.
Molecular Geometry
The bent molecular geometry (approximately 104.5 degrees) is a result of the repulsion between lone pairs of electrons on the oxygen atom. This geometry directly impacts the polarity of the molecule, causing an uneven distribution of charge. This bent shape plays a critical role in influencing water’s interactions with other molecules. It is a fundamental aspect of the molecule’s structure.
Polarity
The polarity of the water molecule, as shown in a water molecular diagram, arises from the uneven distribution of electrons due to oxygen’s higher electronegativity. This polarity allows for hydrogen bonding between water molecules, influencing many of its properties. The dipole moment resulting from polarity is a key feature driving many interactions.
Hydrogen Bonding
Hydrogen bonding, visualized in many water molecular diagrams, is a crucial intermolecular force that arises from the polarity of the molecule. It influences water’s high boiling point, surface tension, and solvent properties. These bonds significantly affect water’s physical and chemical characteristics.
These key aspects, clearly shown in a water molecular diagram, collectively determine the behavior and characteristics of water. The interconnectedness of these aspects highlights the importance of understanding each component for a comprehensive understanding of this vital molecule.
The diagram offers a visual understanding of these properties and their interrelationships, enabling better comprehension of water’s crucial role in various scientific fields and everyday life. Accurate representation is vital for communication and knowledge dissemination. The diagram’s effectiveness lies in its ability to simplify complex concepts.
Tips for Understanding Water Molecular Diagrams
Understanding water molecular diagrams requires attention to detail and a grasp of fundamental chemical concepts. The accurate interpretation of the diagram is paramount in understanding the properties of water. Using various representations can enhance comprehension. Various teaching aids can aid in grasping the concepts presented in the water molecular diagram.
Utilizing different visual representations, such as ball-and-stick models or space-filling models, can improve comprehension of the three-dimensional structure. Relating the diagram to real-world applications, like understanding water’s role as a solvent, enhances understanding. Engaging with interactive learning tools can also strengthen the learning process.
Focus on the atoms and bonds
Pay close attention to the types of atoms (oxygen and hydrogen) and the bonds connecting them. Understanding the nature of covalent bonds is essential for grasping the molecule’s properties. The bond lengths and angles are crucial for determining the overall shape.
Consider the 3D structure
While diagrams are 2D representations, remember water is a three-dimensional molecule. Visualize the molecule in 3D to fully grasp the spatial arrangement of the atoms and the implications of this arrangement.
Understand the polarity
The polarity of water is crucial. Focus on how the unequal sharing of electrons between oxygen and hydrogen atoms creates partial charges, influencing the molecule’s behavior.
Explore different diagram types
Familiarize yourself with different representations such as Lewis dot structures, ball-and-stick models, and space-filling models, each offering unique insights into the molecule’s structure.
Relate the diagram to water’s properties
Connect the features in the diagram (bond angles, polarity, hydrogen bonding) to the unique properties of water, such as its high boiling point and its ability to act as a universal solvent.
The accurate depiction of a water molecular diagram is essential for effectively conveying the molecule’s structure and properties. Mastering the interpretation of such diagrams facilitates a clearer understanding of chemistry and biology. The significance of the water molecular diagram should not be underestimated.
It serves as a powerful tool for education and research, simplifying complex concepts for better comprehension. Various models exist, each useful in conveying different aspects of the molecule’s structure. A thorough understanding of the water molecular diagram enables appreciation for the unique properties and functions of water.
In conclusion, a comprehensive understanding of the water molecular diagram is crucial for grasping the fundamental properties and behaviours of water. Its accurate depiction and interpretation are essential in various scientific and educational settings. Continued learning and exploration of different diagram types enhances the understanding of this vital molecule.
Youtube Video: