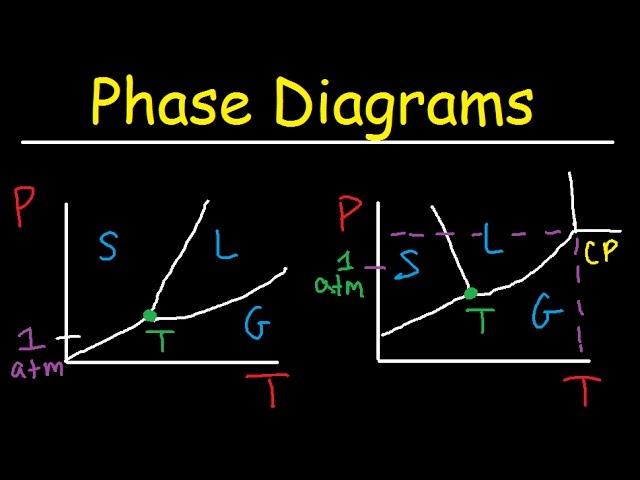
A phase diagram water is a graphical representation of the thermodynamic conditions (temperature and pressure) at which water exists in different phases: solid (ice), liquid (water), and gas (water vapor). Understanding this diagram is crucial for various scientific disciplines and practical applications, providing insights into water’s behavior under diverse conditions. Its construction relies on experimentally determined data points, revealing the equilibrium points between phases. The lines on the diagram represent phase transitions, showing the coexistence of two phases at specific conditions. This simple yet powerful tool is essential for predicting the state of water under a range of environmental scenarios.
The phase diagram water illustrates the transitions between solid, liquid, and gaseous states. At standard atmospheric pressure, water freezes at 0C and boils at 100C. However, the phase diagram reveals that these transition temperatures change significantly with variations in pressure. For instance, under high pressure, the melting point of ice decreases, while at lower pressures, the boiling point decreases. Analyzing a phase diagram water provides a comprehensive understanding of the physical properties of water across its various states and assists in predicting its behavior in different environments. Its applications range from meteorology to material science.
The phase diagram water is not just a static representation; it’s a dynamic tool that offers significant predictive power. By identifying specific temperature and pressure points on the diagram, one can accurately determine the phase water will be in. This knowledge is fundamental for numerous practical uses, including designing industrial processes, understanding atmospheric phenomena, and even predicting the behavior of water in extreme environments like deep oceans or outer space.
Understanding the Phase Diagram Water
The diagram’s most prominent features are the lines separating the different phases. These lines represent conditions where two phases coexist in equilibrium. For example, the line separating the liquid and gas phases represents the boiling point of water at a given pressure. The intersection of all three phase boundaries (solid, liquid, and gas) is known as the triple point, where all three phases can exist simultaneously. This is a unique and highly specific point with defined temperature and pressure values.
Beyond the triple point, the diagram highlights the effects of pressure on the freezing and boiling points of water. Increased pressure typically lowers the freezing point and raises the boiling point, but water exhibits unique behavior near the triple point. This is a result of the unique molecular structure of water and the density differences between ice and liquid water. Careful analysis of these variations is critical for fields like glaciology and oceanography.
-
Gather Data:
Begin by collecting experimental data on the melting, boiling, and sublimation points of water under varying pressures. This often involves meticulous laboratory experiments using controlled environments and precision instruments. Accurate data collection forms the cornerstone of a reliable phase diagram.
-
Plot the Data:
Represent the collected data points on a graph with temperature on the x-axis and pressure on the y-axis. Each point represents a specific temperature and pressure at which a phase transition occurs. Precise plotting is essential for constructing an accurate phase diagram.
-
Draw the Phase Boundaries:
Connect the data points to create lines representing the equilibrium between different phases (solid-liquid, liquid-gas, solid-gas). These lines represent the conditions under which two phases coexist. Carefully labeling these lines is crucial for clarity and understanding.
-
Identify Key Points:
Locate and label the triple point (the point where all three phases coexist) and any other significant points, such as the critical point (beyond which the distinction between liquid and gas ceases). These points offer valuable insights into the thermodynamic properties of water.
Frequently Asked Questions about Phase Diagram Water
The phase diagram water is a frequently used tool in various scientific and engineering disciplines, leading to several common questions regarding its interpretation and application. Understanding the diagram’s nuances is critical for accurate predictions of water’s behavior under different conditions and the ability to apply this knowledge in practical situations. Many questions revolve around the interpretation of the diagram’s lines and points and their implications for specific applications.
What is the significance of the triple point on a phase diagram water?
The triple point represents the unique combination of temperature and pressure where all three phases of watersolid (ice), liquid (water), and gas (water vapor)exist in thermodynamic equilibrium. This specific point is often used as a reference point in various thermodynamic calculations and calibrations. Its significance lies in its unique physical characteristics and the fact that it defines a specific point on the phase diagram, from which the behavior of water at different temperatures and pressures can be predicted.
How does pressure affect the boiling point of water as shown in the phase diagram water?
As pressure increases, the boiling point of water also increases. This is because higher pressure makes it more difficult for water molecules to overcome the intermolecular forces holding them together in the liquid phase, hence requiring a higher temperature to transition to the gas phase. The phase diagram water visually illustrates this relationship, showing the upward slope of the liquid-gas equilibrium line. This principle is utilized in pressure cookers, where increased pressure leads to higher cooking temperatures, thereby shortening cooking times.
How does pressure affect the freezing point of water as depicted in a phase diagram water?
Unlike most substances, the freezing point of water decreases with increasing pressure. This is due to the fact that ice is less dense than liquid water. Increased pressure favors the denser liquid phase, leading to a lower freezing point. This anomalous behavior is clearly shown on the phase diagram water, demonstrating the unique properties of water.
Key Aspects of a Phase Diagram Water
A phase diagram water is a powerful tool in understanding the behaviour of water. Its utility stems from its ability to predict water’s state under given conditions. It is a fundamental concept in various scientific fields. Its application reaches far beyond simple observation.
Pressure
Pressure significantly influences the phase transitions of water. An increase in pressure generally raises the boiling point and lowers the freezing point (though there are exceptions near the triple point). This pressure-dependent behavior is crucial in diverse contexts, such as high-altitude cooking or deep-sea exploration where pressure conditions greatly impact water’s phase.
Temperature
Temperature is another critical factor dictating water’s phase. Changes in temperature drive transitions between solid, liquid, and gas phases. This dependence is fundamental in understanding natural processes like weather patterns, or industrial procedures that involve heating or cooling water.
Phase Transitions
These transitions (melting, freezing, boiling, condensation, sublimation, deposition) are represented by the lines on the diagram. Each line shows the conditions where two phases exist in equilibrium. An understanding of these transitions is essential in numerous fields, from designing industrial processes to analyzing climate change.
Triple Point
This unique point on the diagram represents where solid, liquid, and gas phases coexist in equilibrium. It’s a fixed point used in calibration and thermodynamic studies, highlighting the intersection of all three phase boundaries.
The lines on the diagram demarcate the boundaries between phases. The slopes of these lines indicate the influence of pressure on the phase transitions. The intersection of the three lines defines the triple point. These features help predict the state of water under different conditions.
The phase diagram water is not merely an academic tool; it finds practical application in various fields, including meteorology, climatology, and material science. Its ability to predict water’s state under specific conditions has significant practical implications. This makes it essential for understanding many real-world phenomena.
Tips for Interpreting a Phase Diagram Water
Accurately interpreting a phase diagram water requires a methodical approach. Familiarizing oneself with the axes, understanding the significance of the lines, and recognizing key points are crucial steps in successfully interpreting the diagram and applying this knowledge to real-world scenarios. The diagram’s simplicity belies its importance and predictive capability.
Understanding the relationships between temperature, pressure, and phase is paramount. This knowledge allows for accurate prediction of water’s state under various conditions, expanding our understanding of water’s properties and behavior.
-
Identify the Axes:
Ensure a clear understanding of what each axis represents (temperature and pressure). This is the foundational step for correctly interpreting the data presented on the diagram.
-
Locate the Phase Regions:
Identify the regions representing solid, liquid, and gas phases. These regions are separated by lines representing phase boundaries where two phases coexist.
-
Understand Phase Boundaries:
The lines separating the phase regions illustrate the conditions (temperature and pressure) where phase transitions occur. Knowing how these boundaries shift with pressure changes is vital.
-
Identify the Triple Point:
Locate the triple point, where all three phases (solid, liquid, gas) exist in equilibrium. This special point provides crucial reference data in thermodynamic calculations.
-
Interpret Data Points:
Given a specific temperature and pressure, determine the phase of water using the diagram. This is the most practical application of the phase diagram water.
Mastering the interpretation of a phase diagram water is fundamental to understanding many scientific principles and practical applications. Its importance extends across numerous scientific disciplines. This understanding is crucial for various applications.
The phase diagram water provides a clear, concise, and highly informative visual representation of water’s behavior under different pressure and temperature conditions. Accurate interpretation of this diagram is key to understanding and predicting various phenomena involving water. The utility of this simple yet powerful tool is far-reaching.
In conclusion, the phase diagram water serves as a powerful tool for understanding and predicting the behavior of water under varying conditions. Its application extends beyond theoretical considerations to provide practical insights in various scientific and engineering fields.
Youtube Video: