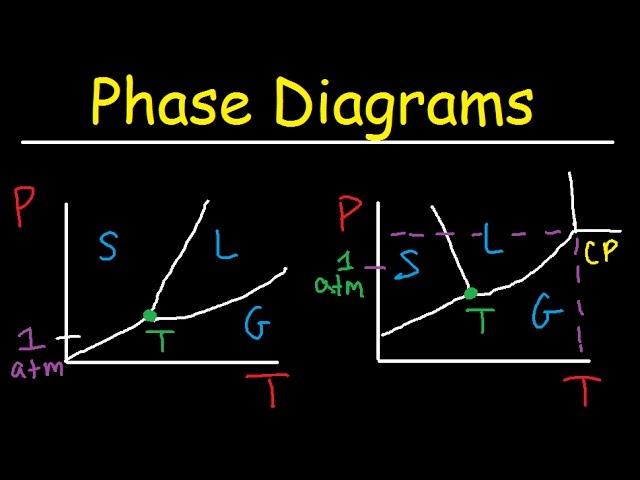
A phase diagram for water is a graphical representation of the thermodynamic conditions (temperature and pressure) at which water exists in different phases solid (ice), liquid (water), and gas (steam). Understanding this diagram is crucial in various scientific fields, from meteorology to materials science, offering insights into the behavior of water under different conditions. The diagram’s curves delineate the boundaries between these phases, illustrating transitions like melting, boiling, and sublimation. Its importance lies in predicting the phase of water under specific environmental parameters. Furthermore, it’s a foundational tool for many scientific studies and engineering applications.
The phase diagram for water displays three distinct regions corresponding to the solid, liquid, and gaseous phases. The lines separating these regions represent phase transitions. For instance, the line separating the liquid and gas phases denotes the boiling point, while the line separating solid and liquid represents the melting point. These points vary with pressure, a key feature highlighted in the diagram. The triple point, where all three phases coexist in equilibrium, is another crucial element. This detailed representation provides a comprehensive understanding of water’s behavior across a range of conditions. Analyzing this diagram allows predictions of phase changes based on temperature and pressure adjustments.
The diagram’s negative slope of the solid-liquid equilibrium line is a unique characteristic of water, resulting in ice being less dense than liquid water. This unusual property has profound implications for aquatic life and the Earth’s climate.
Understanding the Phase Diagram for Water
Analyzing a phase diagram for water involves interpreting the relationships between temperature, pressure, and the phase of water. The diagram’s curves reveal the conditions necessary for phase transitions to occur. For example, increasing the temperature at a constant pressure will lead to a phase transition from solid to liquid and then liquid to gas. Conversely, changing the pressure at a constant temperature can also induce phase transitions. The diagram’s slope illustrates the effect of pressure on melting and boiling points. Its a valuable tool for predicting phase behavior in diverse applications, from industrial processes to understanding natural phenomena.
Beyond its use in predicting phase changes, the diagram also provides insights into the thermodynamic properties of water, such as enthalpy and entropy changes during phase transitions. It offers a visual representation of these often complex relationships, making it a powerful educational tool. The ability to predict phase behavior under varying conditions is critical in various industrial processes, research efforts, and everyday applications. The diagram simplifies these predictions, allowing for efficient design and control of systems involving water.
-
Step 1: Define the axes.
The horizontal axis typically represents temperature, usually in degrees Celsius or Kelvin. The vertical axis represents pressure, often in atmospheres or Pascals. Establishing these axes correctly is the foundation of constructing an accurate diagram. Accurate scaling of both axes is crucial for clear representation of the phase boundaries.
-
Step 2: Plot the phase boundaries.
This involves plotting the lines representing the equilibrium between different phases. The solid-liquid boundary (melting/freezing point), the liquid-gas boundary (boiling/condensation point), and the solid-gas boundary (sublimation/deposition point) need to be accurately marked. Understanding the relationship between pressure and temperature at these transitions is crucial for precise plotting.
-
Step 3: Identify key points.
Mark the triple point (where all three phases coexist) and the critical point (where the distinction between liquid and gas disappears). These points define the extreme conditions within which the three phases can coexist. The accurate placement of these points ensures the diagram’s reliability and predictive power.
Frequently Asked Questions about the Phase Diagram for Water
The phase diagram for water provides a comprehensive visual representation of water’s behavior under different temperature and pressure conditions, answering many crucial questions about its physical properties. Frequently asked questions often revolve around interpreting the diagram to predict phase transitions or understand anomalous behavior of water compared to other substances. The ability to extract meaningful information from this diagram is crucial for various scientific and engineering disciplines.
What is the significance of the negative slope of the solid-liquid equilibrium line in the phase diagram for water?
The negative slope signifies that increasing pressure on ice lowers its melting point. This is unlike most substances where pressure increases the melting point. This unusual behavior stems from the fact that ice is less dense than liquid water due to its hydrogen bonding structure. The reduced density of ice means that applying pressure favors the denser liquid phase, hence the lower melting point at higher pressures.
What happens at the triple point of water on the phase diagram?
At the triple point, all three phases of water solid (ice), liquid (water), and gas (steam) coexist in thermodynamic equilibrium. This is a unique set of temperature and pressure conditions where all three phases can exist simultaneously. This point is a fixed and precisely defined state, serving as a benchmark in various scientific experiments and calibrations.
How does the phase diagram for water help predict the state of water under various conditions?
By locating a given temperature and pressure on the diagram, one can immediately determine the phase of water (solid, liquid, or gas) that will be present under those conditions. The diagram acts as a map, allowing for a quick and accurate prediction of water’s phase behavior based on its environmental parameters. This is crucial in fields like meteorology, where understanding the conditions for different states is vital for weather forecasting.
The diagram visually represents the transitions between the solid, liquid, and gaseous states of water. These transitions are dictated by changes in temperature and pressure, clearly illustrated on the phase diagram. Understanding the diagram allows one to predict the phase of water under specific conditions, a feature that proves invaluable in various scientific and engineering applications.
Careful interpretation of the phase diagram provides critical information for various applications. The lines and points on the diagram represent specific transitions and conditions where multiple phases coexist. Using the diagram efficiently requires understanding how temperature and pressure influence the phase of water.
Key Aspects of the Phase Diagram for Water
The phase diagram for water is a critical tool, offering insights into its unique behavior and providing a predictive framework. Its key aspects highlight the influence of pressure and temperature on water’s physical state transitions. Comprehensive understanding allows for accurate predictions of water’s behavior in various environments.
Temperature Dependence
Temperature significantly impacts the phase of water, transitioning from solid to liquid to gas with increasing temperature at standard pressure. This dependence is linear at constant pressure but changes dramatically near phase transition points. This predictable behavior is vital for understanding numerous natural phenomena.
Pressure Dependence
Pressure also influences water’s phase, altering melting and boiling points. Understanding the pressure dependence is crucial in high-pressure applications and for understanding water’s behavior in deep ocean environments. A pressure increase can influence phase transitions in a non-linear manner near the critical point.
Triple Point
The unique triple point represents the single set of temperature and pressure conditions where all three phases (solid, liquid, gas) coexist in equilibrium. This specific point is a crucial reference point for calibrations and thermodynamic studies. Its fixed nature provides a stable benchmark for various measurements.
Critical Point
At the critical point, the distinction between liquid and gas phases disappears. This specific point represents the maximum temperature and pressure where a liquid-gas transition can be observed. Understanding the critical point is vital for processes involving supercritical fluids.
Understanding these key aspects allows for accurate predictions of water’s phase behavior under diverse conditions. The diagram’s ability to simplify complex relationships between temperature, pressure, and phase transitions makes it a valuable tool across scientific and engineering disciplines.
The phase diagram provides a visual representation of these dependencies, allowing for easy visualization and interpretation. Accurate interpretation of the diagram is crucial for accurately predicting water’s state under varied pressure and temperature conditions.
Tips for Interpreting the Phase Diagram for Water
Effectively utilizing a phase diagram for water requires careful interpretation and understanding of its key elements. Practicing interpretation skills will improve one’s comprehension of the relationship between temperature, pressure, and water’s state transitions. This knowledge enhances problem-solving capabilities in various scientific and engineering fields.
To avoid misinterpretations, pay close attention to the axes and units of measurement. The accurate understanding of scales is critical for correct interpretation of phase transitions. Remember that each line represents a unique state transition.
Tip 1: Understand the axes.
Ensure you comprehend the scales used for temperature and pressure. Accurate interpretation hinges on a clear understanding of these scales. Inconsistent or unclear scales can lead to inaccurate conclusions. Knowing the units (e.g., Celsius, Kelvin; atmospheres, Pascals) is crucial.
Tip 2: Identify the regions.
Clearly identify the regions representing the solid, liquid, and gaseous phases. Each region corresponds to the dominant phase of water under the specified conditions. Differentiating regions accurately allows for precise phase predictions.
Tip 3: Interpret the lines.
Understand that the lines separating the regions represent phase transitions. These lines show the pressure-temperature combinations at which phase transitions occur. The slopes of these lines reflect the sensitivity of phase transitions to changes in pressure.
Tip 4: Locate the triple point.
Understand the significance of the triple point, where all three phases coexist. This point represents a unique set of thermodynamic conditions. Knowing the coordinates of this point is essential for understanding waters behavior under extreme conditions.
Tip 5: Locate the critical point.
Recognize the critical point, where the liquid-gas distinction vanishes. This is a significant point, marking the limits of the liquid-gas phase boundary. Understanding its implications is crucial for interpreting phase behavior under extreme pressures and temperatures.
The phase diagram for water serves as a powerful tool for understanding the physical properties and behavior of water under various conditions. It provides a visual and quantitative approach to comprehending how temperature and pressure influence phase changes. Mastering its interpretation is essential for diverse scientific and engineering disciplines.
The ability to predict the phase of water based on temperature and pressure is critical in numerous applications. This predictive capability is a direct consequence of the informative nature of the phase diagram. Thorough understanding of the diagram enables the solving of various problems related to water’s physical state.
In conclusion, a comprehensive understanding of the phase diagram for water is crucial for various scientific and engineering applications. Its ability to visually represent the complex relationships between temperature, pressure, and phase transitions makes it an indispensable tool. Mastering the interpretation of this diagram empowers a deeper understanding of water’s behavior under diverse conditions.
Youtube Video: