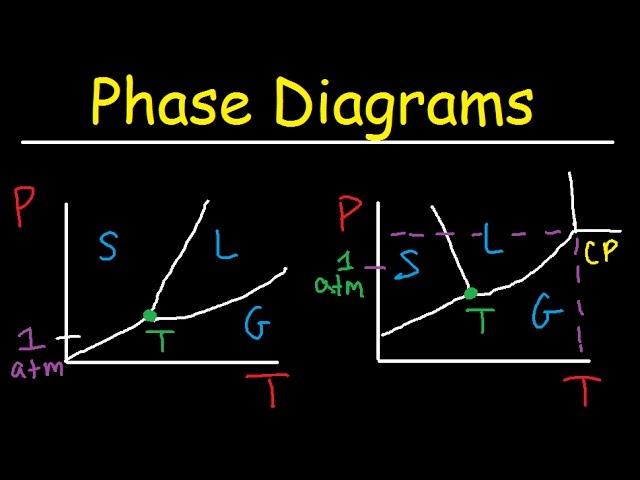
An HO phase diagram is a graphical representation of the physical states of water (solid, liquid, and gas) as a function of temperature and pressure. This diagram is crucial for understanding the behavior of water under various conditions, from the freezing point at sea level to the boiling point at high altitudes. It highlights the transitions between phases and reveals the existence of unique points such as the triple point and the critical point. The diagram’s simplicity belies its importance in fields ranging from meteorology to chemistry and material science. It allows for predictions about the state of water given specific temperature and pressure values.
The HO phase diagram illustrates the equilibrium conditions between the different phases of water. A line on the diagram represents the conditions where two phases coexist in equilibrium for instance, the line separating the liquid and gas phases represents the boiling point at various pressures. The area within the diagram shows the range of conditions where a single phase (solid, liquid, or gas) is stable. Examining this diagram provides valuable insights into water’s behavior under diverse environmental pressures and temperatures. Understanding this helps to predict phenomena like ice formation, evaporation, and the behavior of water in extreme environments.
Analyzing a phase diagram helps understand how pressure and temperature affect water’s state. For example, it demonstrates that ice can sublime directly into water vapor under low-pressure conditions, bypassing the liquid phase. Also, it reveals how high pressure can influence the melting point of ice, leading to its melting at a temperature lower than 0C. The significance of the diagram lies in its power to provide a visual overview of water’s thermodynamic properties, facilitating a better grasp of its versatile nature in diverse applications.
Understanding the HO Phase Diagram
The diagram’s most notable features include the triple point, where all three phases coexist, and the critical point, beyond which the distinction between liquid and gas disappears. The slopes of the phase boundaries reveal important thermodynamic information about water’s properties. For example, the negative slope of the solid-liquid boundary signifies that ice is less dense than liquid water, a unique characteristic with significant implications for aquatic life and Earth’s climate. The diagram helps analyze phenomena such as melting, boiling, and sublimation, emphasizing how these processes are influenced by pressure and temperature.
Detailed analysis reveals important relationships between pressure, temperature, and phase transitions. By studying the diagram, scientists and engineers can predict the behavior of water in various applications, from designing efficient cooling systems to understanding atmospheric processes. The information derived from the diagram is essential for various scientific and engineering fields. This leads to improved designs in industrial and technological sectors, contributing greatly to practical applications.
-
Determine the axes:
The horizontal axis represents temperature, usually in degrees Celsius or Kelvin, and the vertical axis represents pressure, typically in atmospheres or Pascals. The accurate scaling is crucial for correct interpretation.
-
Identify the regions:
Each region of the diagram represents a specific phase of water: solid (ice), liquid (water), and gas (water vapor). Understanding the boundaries between these regions is key.
-
Locate the phase boundaries:
Lines separating the regions indicate the conditions at which two phases coexist in equilibrium. These lines represent phase transitions like melting, boiling, and sublimation.
-
Examine special points:
The triple point, where all three phases coexist, and the critical point, where the distinction between liquid and gas vanishes, are particularly important points on the diagram.
Frequently Asked Questions about the HO Phase Diagram
The HO phase diagram is a powerful tool, but understanding its intricacies can raise questions. Many aspects, from interpreting its features to applying its insights to real-world situations, can lead to inquiries. Addressing these questions will provide a more comprehensive understanding of this crucial diagram and its applications. Clear answers help in appreciating the value of this scientific tool, applicable in various scientific disciplines and industries.
What is the significance of the negative slope of the solid-liquid boundary in the HO phase diagram?
The negative slope signifies that increasing pressure on ice lowers its melting point. This is because ice is less dense than liquid water. This unusual property has significant implications for various phenomena, including the gliding of glaciers and the behavior of ice under pressure. The implications extend to biological systems and the geological processes influenced by water’s unique properties.
How does the HO phase diagram help explain the sublimation of ice?
The diagram shows that at low pressures, ice can directly transition to water vapor without passing through the liquid phase. This sublimation process is evident in the region where the solid and gas phases meet. This understanding has practical applications, such as freeze-drying and the preservation of materials.
What is the triple point and its importance?
The triple point is the unique condition of temperature and pressure where all three phases of water solid, liquid, and gas coexist in thermodynamic equilibrium. Its precise values are used to define temperature scales and are significant for calibrating scientific instruments and thermodynamic calculations.
The HO phase diagram is not merely a static image; it represents a dynamic interplay of temperature and pressure. The transitions between phases are governed by the specific energy levels and molecular interactions within the water substance. Understanding this allows us to make accurate predictions and extrapolations based on the principles illustrated within the diagram.
Furthermore, the information encapsulated within the diagram provides a foundation for advanced thermodynamic concepts. Its insights are essential for the development of new technologies and the improvement of existing ones. The diagram serves as a cornerstone of understanding across various disciplines.
The diagram also highlights the importance of considering both temperature and pressure when discussing the state of water. These parameters are not independent but rather are interconnected in their influence on the phases of water. This interconnectedness has broad implications for a wide variety of applications.
Key Aspects of the HO Phase Diagram
The diagram’s value lies in its ability to concisely portray complex relationships. Analyzing it unveils critical information about waters physical states and transitions under different conditions. This makes it a cornerstone tool in multiple scientific and engineering domains. The diagram’s utility spans theoretical understanding to practical applications.
Phase Transitions
The diagram visually depicts the transitions between solid, liquid, and gaseous states, illustrating the processes of melting, freezing, boiling, condensation, and sublimation. These transitions are crucial to understanding water’s behavior in various systems.
Pressure Influence
The diagram demonstrates how changes in pressure affect the melting and boiling points of water. This has implications for various industrial applications and natural phenomena.
Temperature Dependence
The diagram showcases how temperature influences waters phase. This fundamental relationship is central to countless processes within natural and manufactured systems.
Triple Point
This unique point on the diagram marks the specific pressure and temperature at which all three phases of water coexist. It’s a crucial reference point in thermodynamics.
Critical Point
Beyond the critical point, the distinction between liquid and gas phases disappears. This understanding has applications in high-pressure systems and advanced research.
Each aspect, from the distinct phases to the critical and triple points, offers insights into water’s complex behavior and its interaction with environmental factors. Together, they paint a comprehensive picture of how this fundamental substance changes under varying conditions.
The diagrams efficacy is reinforced by its simplicity and accessibility. Despite its visual representation, it conveys complex thermodynamic relationships clearly and concisely. This enhances its utility across diverse fields and applications.
Tips for Understanding the HO Phase Diagram
Mastering the interpretation of the HO phase diagram requires a strategic approach. Understanding its nuances enables accurate prediction of water’s phase under specific conditions. This knowledge benefits various scientific and engineering disciplines.
Effective utilization involves grasping the relationship between pressure, temperature, and phase. This understanding enables accurate interpretation of the diagram and its applications. This crucial knowledge is fundamental across many scientific and technological fields.
Start with the basics:
Familiarize yourself with the concepts of temperature, pressure, and the three phases of water. This foundational knowledge forms the basis for understanding the diagram’s representations.
Focus on the axes:
Understand how temperature and pressure are represented on the diagram’s axes. Their interaction determines the water’s phase.
Identify the regions:
Clearly delineate the areas representing solid, liquid, and gaseous phases. This visual identification is crucial for interpreting the diagrams message.
Examine the boundaries:
Carefully study the lines separating the phases. These boundaries represent phase transitions and their associated conditions.
Analyze special points:
Pay close attention to the triple and critical points. Their presence signifies unique thermodynamic conditions.
Practice interpreting:
Use the diagram to predict the state of water under various pressure and temperature conditions. Practice is essential for mastery.
The HO phase diagram is more than just a scientific tool; it’s a visual representation of the fundamental principles governing water’s behavior. Understanding its intricacies provides insights into a range of natural phenomena and has practical applications in numerous technological processes.
Beyond its immediate applications, the phase diagram serves as a powerful example of how a simple visual representation can effectively convey profound scientific principles. It underscores the importance of visualizing data for enhanced understanding.
In conclusion, a thorough understanding of the HO phase diagram is crucial for anyone working with water in various contexts, from scientists conducting research to engineers designing industrial processes. Its application extends far beyond simple academic exercises and holds profound implications for technological innovation and fundamental scientific knowledge.
Therefore, a comprehensive grasp of the HO phase diagram is essential for a wide range of scientific and engineering endeavors, contributing to both fundamental understanding and practical applications.
Youtube Video: