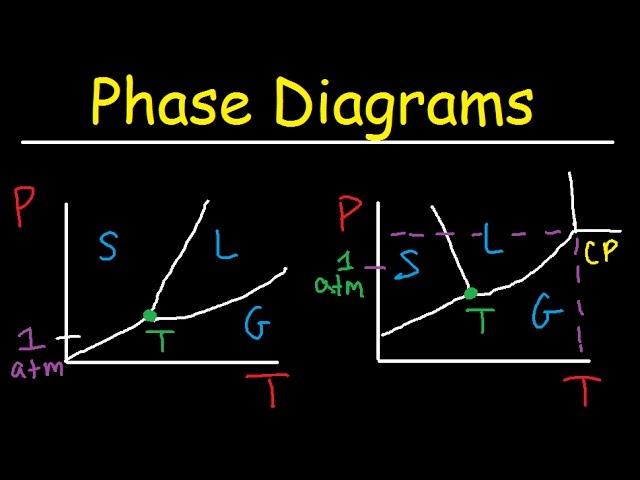
A CO2 phase diagram is a graphical representation of the thermodynamic conditions at which carbon dioxide exists in different phases solid, liquid, and gas. Understanding this diagram is crucial for various applications, from carbon capture and storage to the design of refrigeration systems. The diagram illustrates how pressure and temperature influence the phase transitions of CO2, providing a visual tool for predicting its behavior under different conditions. Its accurate depiction of these transitions is essential for numerous industrial processes and scientific research. The unique properties of CO2, particularly its supercritical state, are clearly highlighted on the diagram.
The CO2 phase diagram is not simply a static image; it’s a dynamic representation of CO2’s behavior. Changes in pressure and temperature can move a system from one phase to another, as shown by the lines on the diagram that delineate these phase boundaries. The triple point, where all three phases coexist in equilibrium, is a particularly important feature. Similarly, the critical point marks the boundary beyond which the distinction between liquid and gas becomes meaningless, resulting in a supercritical fluid. The CO2 phase diagrams ability to visualize these transitions provides a critical framework for diverse applications. This enables scientists and engineers to predict the behavior of CO2 in various scenarios, ensuring safe and efficient operation of many systems.
Understanding the CO2 Phase Diagram
The pressure-temperature relationship depicted in the CO2 phase diagram allows for precise prediction of CO2’s state under specific conditions. By knowing the pressure and temperature, one can determine whether the CO2 will be a solid (dry ice), a liquid, a gas, or in a supercritical state. This predictability is vital in various industrial settings. For example, in carbon capture and storage, accurately predicting the CO2 phase is critical for safe and efficient transportation and underground sequestration. The diagram also informs the design of processes that exploit the unique properties of supercritical CO2, such as its increased solvent power compared to liquid CO2. Analyzing the diagram allows for optimizing numerous industrial applications.
A thorough understanding of the phase diagram is essential for engineers and scientists working with CO2, from designing equipment for its handling and storage to researching its various applications. The diagram helps to avoid potential hazards associated with phase transitions, such as pressure buildup or uncontrolled changes in state. Moreover, it aids in the optimization of CO2-based processes, leading to improved efficiency and reduced waste. The accurate prediction of the CO2 phase, facilitated by the diagram, is critical for successful and sustainable industrial applications.
-
Determine the pressure and temperature:
First, identify the pressure and temperature of the CO2 system you are considering. This might be from direct measurement or from a process model. Accurate data input is paramount for a reliable result. Ensure the units are consistent and appropriate for the diagram being used. The accuracy of the prediction directly relies on the accuracy of these values.
-
Locate the point on the diagram:
Using the pressure and temperature values, locate the corresponding point on the CO2 phase diagram. This point represents the thermodynamic state of your system. Careful plotting is essential, as slight variations can alter the interpreted phase. Use appropriate tools and techniques to ensure precision in locating the point on the phase diagram.
-
Identify the phase region:
Once the point is located, determine the phase region (solid, liquid, gas, or supercritical) in which it falls. This region indicates the predominant phase of CO2 under the specified conditions. Understanding the boundaries between these regions is key to interpreting the phase diagram effectively. Consult a properly annotated diagram for accurate identification of the phase regions.
Frequently Asked Questions about the CO2 Phase Diagram
The CO2 phase diagram, while seemingly simple, can generate many questions regarding its interpretation and application. Common queries often center around the significance of specific points on the diagram, such as the triple and critical points, and the implications of crossing phase boundaries. Others involve practical applications, such as predicting CO2 behavior in various industrial processes. Understanding the answers to these questions is paramount for effective utilization of the diagram in a variety of fields.
What is the significance of the triple point on the CO2 phase diagram?
The triple point represents the unique condition of temperature and pressure where solid, liquid, and gaseous CO2 coexist in thermodynamic equilibrium. This point is a fixed reference point, defined by precise values of temperature and pressure, and is used as a calibration point in various experiments and simulations. It is a characteristic property of CO2 and has practical implications in various applications involving CO2 phase transitions. Its significance lies in the demonstration of a simultaneous equilibrium between three distinct phases.
What is a supercritical fluid, and how is it shown on the CO2 phase diagram?
A supercritical fluid is a state of matter beyond the critical point on the CO2 phase diagram, where the distinction between liquid and gas phases disappears. Supercritical CO2 possesses unique properties, such as high density and solvent power, which make it valuable in various industrial applications, such as extraction and cleaning processes. The CO2 phase diagram clearly visualizes this unique region and its boundaries with liquid and gas regions, highlighting its importance in different processes. Supercritical CO2 shows unique solvent properties compared to liquid or gaseous states.
The CO2 phase diagram provides a valuable tool for understanding the phase behavior of carbon dioxide under various conditions. Its accurate use requires a thorough understanding of pressure-temperature relationships and phase transitions. Misinterpretation of the diagram can lead to inaccurate predictions and potential safety risks. Proper interpretation and application of this diagram are essential in different scientific and industrial contexts.
The utility of the CO2 phase diagram extends far beyond simple phase identification. Its application allows for the optimization of processes involving CO2, enhancing efficiency and safety. Accurately predicting the CO2 phase is critical in many industries, leading to better resource management and reduced environmental impact. This ensures optimal conditions are maintained for the process involved, thereby maximizing efficiency.
Key Aspects of the CO2 Phase Diagram
The CO2 phase diagram, as a noun, encompasses several key aspects vital for its comprehension and application. These aspects highlight its importance in various scientific and industrial processes and provide insights into its predictive capabilities. These aspects illustrate its function as a tool for understanding and controlling the behavior of CO2 under changing thermodynamic conditions.
Pressure-Temperature Relationship
This fundamental aspect showcases how pressure and temperature influence CO2’s phase. Higher pressure favors the liquid or solid phase, while higher temperature promotes the gaseous phase. The interactive nature of pressure and temperature on CO2 phase transition is crucial and is precisely represented on this diagram. This relationship is central to understanding and predicting CO2 behavior under varying thermodynamic conditions.
Phase Boundaries
These lines on the diagram define the conditions at which phase transitions occur. Crossing a boundary indicates a change from one phase to another. This visualization of phase boundaries helps determine the exact conditions that influence the transition and provides insight into the system’s response. The precise location of these boundaries is crucial for accurate phase predictions and process optimization.
Triple Point
This unique point signifies the coexistence of solid, liquid, and gas phases in equilibrium. It marks a specific pressure and temperature combination representing a critical state for CO2. The accurate determination of this point helps in calibration and validation of related experimental data. It provides a fixed reference point for understanding the intricate interactions between phases.
Critical Point
This point represents the upper limit of the liquid-gas coexistence region. Beyond this point, supercritical CO2 exists, possessing unique properties. Understanding this transition is crucial for applications utilizing supercritical CO2 for solvent applications. The existence of this point highlights the distinctive behavior of CO2 beyond typical liquid-gas equilibrium.
These key aspects demonstrate the power of the CO2 phase diagram as a predictive tool. By understanding these elements, one can accurately determine the state of CO2 under specific conditions, enabling better design and control of various processes involving CO2.
Moreover, the CO2 phase diagram’s ability to predict the phase behavior allows for optimal design of equipment and processes. This leads to increased efficiency, reduced waste, and enhanced safety. The avoidance of unexpected phase transitions is crucial for safe operation of CO2-handling systems.
Tips for Utilizing the CO2 Phase Diagram
Effectively using a CO2 phase diagram requires a clear understanding of its interpretation and application. Several key tips can improve accuracy and prevent misinterpretations. Remembering these points will result in better utilization of the diagram for practical purposes, improving decision-making and optimizing various processes.
Careful attention to detail is crucial when using the diagram, as small errors in pressure or temperature readings can lead to significant misinterpretations of CO2’s phase. Using accurate measurement instruments and precise plotting techniques are key elements to obtaining a reliable result.
Always check the units:
Ensure consistent units (e.g., kPa for pressure, C for temperature) throughout your calculations and the diagram’s axes. Inconsistent units can lead to incorrect phase predictions. Conversion factors should be used accurately to maintain consistency.
Accurately locate the point:
Precisely plot the pressure and temperature values on the diagram to avoid misinterpretation of the phase region. Carefully align the coordinates for accurate placement of the data point on the graph.
Understand the phase boundaries:
Clearly identify the boundaries between solid, liquid, gas, and supercritical regions. Recognizing these boundaries is vital in determining the phase at a given pressure and temperature. A well-annotated diagram is highly recommended for this purpose.
Consider the limitations:
Remember that the diagram is a simplified representation. Real-world systems may deviate slightly from the ideal conditions shown. External factors can influence phase behavior. These deviations should be considered when interpreting the diagram.
Consult multiple sources:
Use multiple reputable sources to confirm the accuracy of the diagram and its interpretation. This corroboration helps ensure the accuracy and reliability of the data used for analysis. Use well-established scientific resources for accurate diagrams and information.
The CO2 phase diagram is a fundamental tool for understanding and predicting the behavior of carbon dioxide. Its accurate interpretation is essential for various scientific and industrial applications, from designing efficient carbon capture systems to optimizing processes that use supercritical CO2. Accurate use prevents costly mistakes and safety hazards.
Mastering the CO2 phase diagram empowers scientists and engineers to make informed decisions, leading to safer, more efficient, and more sustainable industrial processes. Through careful application and interpretation of this diagram, significant advancements can be achieved in various fields involving CO2.
In conclusion, a thorough understanding of the CO2 phase diagram is paramount for anyone working with carbon dioxide. Its application provides essential insights for optimizing various processes and minimizing potential hazards. This versatile tool helps in understanding and predicting the behavior of CO2, crucial for numerous applications. The proper utilization of this diagram ensures safer and more efficient processes across different industrial and scientific fields.
Youtube Video: