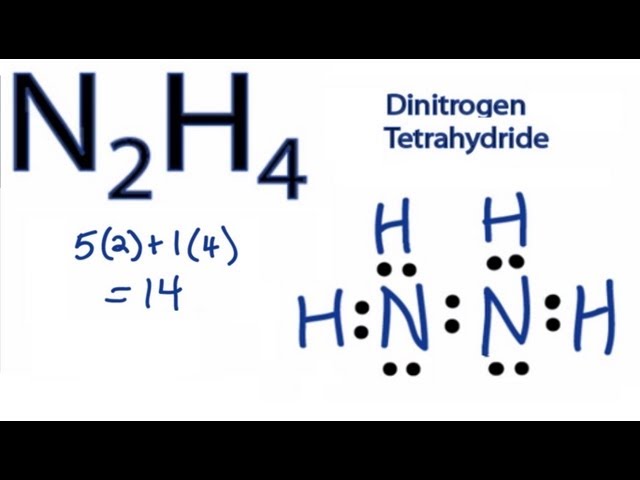
A Lewis diagram for NH, also known as hydrazine, is a visual representation of its valence electrons and bonding structure. This diagram is crucial for understanding the molecule’s properties and reactivity. It shows the arrangement of atoms and the distribution of electrons, highlighting the covalent bonds and lone pairs. Constructing and interpreting this diagram provides valuable insights into hydrazine’s chemical behavior. The simple yet powerful nature of this representation makes it a cornerstone of introductory chemistry.
The Lewis diagram for NH illustrates the connectivity of the two nitrogen atoms and the four hydrogen atoms. Each nitrogen atom possesses five valence electrons, while each hydrogen atom contributes one. To achieve a stable octet configuration for each nitrogen, covalent bonds are formed between the nitrogen atoms and the hydrogen atoms. Lone pairs of electrons are also present on the nitrogen atoms. Understanding this electron distribution is essential for predicting the molecule’s geometry and polarity.
Creating a Lewis diagram for NH involves systematically accounting for all valence electrons and arranging them to minimize formal charges. The process highlights the importance of electron pairs in chemical bonding and molecular stability. The resulting diagram acts as a fundamental building block for understanding more complex chemical concepts related to hydrazine.
Constructing a Lewis Diagram for NH
The construction of the Lewis structure is a methodical process. First, the total number of valence electrons needs to be calculated, summing the contributions from each nitrogen and hydrogen atom. Next, these electrons are arranged to satisfy the octet rule for nitrogen, with hydrogen forming only one bond. Finally, any remaining electrons are placed as lone pairs on the nitrogen atoms. The resulting structure demonstrates the bonding within the hydrazine molecule.
The resulting structure provides a clear visual representation of hydrazines bonding, facilitating the prediction of its molecular geometry and reactivity. The process underscores the key role of valence electrons in determining molecular structure and chemical behavior. Further analysis can reveal details about bond angles and polarity, which are essential in understanding hydrazine’s physical and chemical properties.
-
Determine the total number of valence electrons:
Nitrogen has 5 valence electrons each (2 x 5 = 10 electrons), and hydrogen has 1 valence electron each (4 x 1 = 4 electrons). The total is 14 valence electrons.
-
Identify the central atom(s):
In this case, both nitrogen atoms are central. They are bonded to each other and to hydrogen atoms. This arrangement ensures that each atom, where possible, achieves a stable octet.
-
Connect atoms with single bonds:
Connect the two nitrogen atoms with a single bond, using two electrons. Then connect each nitrogen to two hydrogen atoms using single bonds, consuming a further six electrons.
-
Complete octets:
Add the remaining electrons (14 – 8 = 6 electrons) as lone pairs on the nitrogen atoms to complete their octets. Each nitrogen atom will have one lone pair of electrons.
Frequently Asked Questions about a Lewis Diagram for NH
Understanding the Lewis diagram for NH is fundamental to comprehending the behavior of this important chemical compound. Many questions arise regarding its construction and interpretation, highlighting the significance of this simple yet powerful representation in understanding molecular structure and reactivity. The following FAQ section aims to address these common queries and enhance understanding of this crucial concept in chemistry.
What is the molecular geometry of NH based on its Lewis diagram?
The Lewis diagram shows that each nitrogen atom in NH has four electron groups around it (one N-N bond, two N-H bonds and one lone pair). This corresponds to a tetrahedral electron geometry around each nitrogen atom. However, the molecular geometry, considering only the positions of the atoms, is slightly distorted tetrahedral due to the lone pairs on the nitrogen atoms. These lone pairs exert a stronger repulsive force and therefore influence the bond angles, leading to a less-than-ideal tetrahedral angle.
Why is the Lewis diagram important for predicting hydrazines properties?
The Lewis structure reveals the electron distribution in hydrazine, showing lone pairs on nitrogen and the covalent bonds. This directly informs predictions about several properties such as bond angles, polarity (because of the presence of polar N-H bonds and lone pairs), and reactivity (which is affected by the presence of lone pairs, making it a good nucleophile, and the N-N bond, which is relatively weak). Knowing the electronic structure allows us to understand interactions with other substances.
How does the Lewis diagram help understand hydrazine’s reactivity?
The Lewis diagram clearly shows the lone pairs of electrons on the nitrogen atoms. These lone pairs are readily available for donation to electron-deficient species. This makes hydrazine a good nucleophile, meaning it readily participates in reactions where it donates electrons. The relative weakness of the N-N bond also contributes to its reactivity; this bond is susceptible to cleavage under various reaction conditions.
Key Aspects of the NH Lewis Diagram
Analyzing the Lewis diagram for NH reveals several crucial aspects of its structure and reactivity. These aspects provide a comprehensive understanding of this important compound and its implications in chemistry. Understanding these key features is fundamental to studying its applications and interactions in various chemical processes.
Valence Electrons
The total number of valence electrons (14) dictates the possible bonding arrangements and the presence of lone pairs. This directly impacts the molecular geometry and reactivity. The arrangement of these electrons is the core focus of the Lewis structure, determining the molecule’s overall bonding pattern and its consequent behavior. This foundational aspect underpins all subsequent interpretations and analyses of the compound’s behavior.
Octet Rule
The fulfillment of the octet rule for nitrogen atoms (except for the rare cases of hypervalency) is paramount in creating a stable structure. It forms the basis of the diagram and explains the molecules preference for covalent bonding arrangements. Any deviation from the octet rule implies an unusual behavior in the molecule, suggesting possible instability or specific reactivity patterns. The fulfillment of this rule is essential for the stability and overall behavior of the hydrazine molecule.
Lone Pairs
The lone pairs of electrons on the nitrogen atoms significantly influence the molecule’s shape (distorted tetrahedral) and reactivity (making it nucleophilic). These non-bonding electrons are essential in determining hydrazine’s interactions with other molecules and its participation in different chemical reactions. The presence of lone pairs is directly related to the molecules capacity to form coordinate bonds or act as Lewis bases.
Bonding
The single bonds between the nitrogen atoms and the nitrogen-hydrogen bonds dictate the overall structure. The nature and strength of these bonds significantly influence the molecules physical and chemical properties, including its reactivity and stability. This element of the diagram is critical in predicting the molecules overall behavior in different chemical environments.
These key aspects work together to define hydrazines unique chemical characteristics. Each component plays a crucial role in explaining hydrazines behavior. The interconnectedness of these components is central to a complete understanding of hydrazines chemistry.
Careful examination of the diagram reveals not only the connectivity of atoms but also the distribution of electrons, which are fundamental to understanding its properties and reactivity.
Tips for Drawing a Lewis Diagram for NH
Drawing an accurate Lewis diagram for NH requires a systematic approach and a clear understanding of valence electrons and the octet rule. It is crucial to follow established guidelines and techniques to obtain a correct representation of the molecule’s electronic structure. This ensures proper understanding and effective predictions regarding the molecules behavior and reactions. This section provides helpful steps and considerations that can facilitate the process of constructing the Lewis diagram for NH.
A methodical process ensures the accuracy of the structure, essential for predicting the molecule’s properties and behavior. Adhering to these tips minimizes the risk of errors and facilitates a clear understanding of the bonding within hydrazine.
Count Valence Electrons
Accurately determine the total number of valence electrons contributed by each atom. This forms the foundation for constructing the structure. Without this precise count, the remaining steps are prone to error.
Arrange Atoms
Place the atoms in a logical arrangement, typically with the least electronegative atom in the center. This positioning ensures a structurally reasonable representation of the compound. This step requires prior knowledge of electronegativity and bonding preferences.
Form Single Bonds
Connect atoms with single bonds, ensuring the consumption of electron pairs. This stage creates the backbone of the Lewis diagram, upon which the remaining electron distribution is constructed. This requires understanding the fundamental concept of chemical bonds.
Add Lone Pairs
Add any remaining electrons as lone pairs to complete the octets of the atoms. This step is critical in determining the overall molecular shape and the possibility of additional reactions. The addition of these lone pairs is directly related to the molecules capacity to form coordinate bonds.
Check Formal Charges
Verify the formal charges of each atom to assess the stability of the structure. Minimizing formal charges suggests a more stable and therefore more plausible configuration of the molecule. This step involves knowledge of the formal charge calculation method.
Consider Resonance (if applicable)
If multiple valid Lewis structures are possible, determine the resonance structures. This aspect is vital in portraying an accurate representation of the molecule when considering electron delocalization. Understanding resonance contributes to a complete depiction of the electronic structure.
Creating a Lewis structure is a fundamental skill in chemistry. Mastering this skill enhances ones ability to predict a molecules geometry, reactivity, and other properties. The careful application of this knowledge forms the basis for a thorough understanding of the chemical world.
Understanding the Lewis diagram for NH is crucial for predicting its behavior in chemical reactions. Its accurate construction involves following well-defined steps and requires a thorough understanding of electron arrangements and bonding principles.
In conclusion, the Lewis diagram for NH serves as a powerful tool for visualizing and understanding the molecular structure and properties of hydrazine. Its importance in chemistry extends from fundamental bonding concepts to advanced applications, including the prediction of its reactivity and interactions in various chemical settings.
Youtube Video: