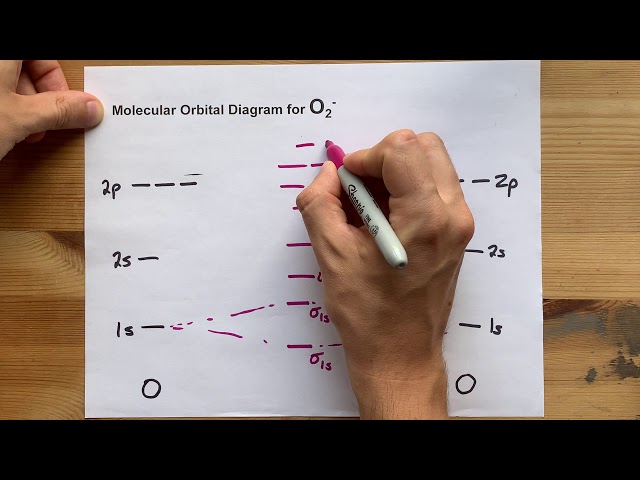
A molecular orbital (MO) diagram for O2 provides a visual representation of the electronic structure of the oxygen molecule. This diagram illustrates how atomic orbitals combine to form molecular orbitals, explaining the molecule’s bond order, magnetic properties, and overall stability. Understanding the MO diagram for O2 is crucial for comprehending its reactivity and behavior in various chemical processes. The diagram reveals the presence of unpaired electrons, accounting for oxygen’s paramagnetism. Constructing and interpreting this diagram relies on fundamental principles of molecular orbital theory.
The MO diagram for O2 is constructed by combining the atomic orbitals of two oxygen atoms. Each oxygen atom contributes two 2s and four 2p atomic orbitals. Upon combination, these atomic orbitals form molecular orbitals (2s, 2s, 2p, 2p, 2p, 2p). The resulting molecular orbitals are filled according to the Aufbau principle and Hund’s rule, leading to a specific electron configuration. The MO diagram for O2 showcases the energy levels and occupancy of these molecular orbitals. This detailed electron distribution is essential for understanding oxygen’s chemical properties.
The MO diagram for O2 accurately predicts the oxygen molecule’s paramagnetism due to the presence of two unpaired electrons in the degenerate 2p antibonding orbitals. The bond order, calculated as half the difference between bonding and antibonding electrons, determines the stability of the bond. The diagram provides a quantitative measure of this stability and allows for predictions of the molecule’s reactivity. This information is critical in various fields, including chemistry, materials science, and atmospheric science.
Constructing the MO Diagram for O2
Creating an accurate MO diagram requires a systematic approach. First, the atomic orbitals of the oxygen atoms are considered, specifically the valence orbitals (2s and 2p). These orbitals are then combined linearly to generate molecular orbitals, leading to bonding and antibonding orbitals. The energies of these molecular orbitals are determined based on their interactions, which in turn is influenced by the symmetry of the atomic orbitals and overlap integrals. Electron configuration is subsequently deduced based on the Aufbau principle and Hund’s rule, finally yielding the complete MO diagram for O2.
The resulting MO diagram visually represents the energy levels of the molecular orbitals and their electron occupancy. The relative energies of the 2p and 2p orbitals are crucial, and determining the correct ordering requires careful consideration of the atomic orbital interactions. This process often involves advanced computational techniques for more accurate energy level predictions. The resulting electron configuration dictates several properties of O2 like its bond order and magnetic susceptibility. Understanding this process is essential for interpreting the diagram effectively.
-
Identify Valence Orbitals:
Begin by identifying the valence atomic orbitals of oxygen (2s and 2p). Consider the number of electrons contributed by each oxygen atom.
-
Combine Atomic Orbitals:
Linearly combine the atomic orbitals to form molecular orbitals. This leads to the formation of sigma () and pi () bonding and antibonding orbitals ( , ).
-
Determine Orbital Energies:
Determine the relative energies of the formed molecular orbitals. This step often requires advanced computational methods for precision, although a qualitative approach using symmetry arguments is also possible.
-
Fill Molecular Orbitals:
Fill the molecular orbitals with electrons according to the Aufbau principle (lowest energy levels first) and Hund’s rule (maximizing electron spin).
-
Calculate Bond Order:
Calculate the bond order using the formula: (Number of electrons in bonding orbitals – Number of electrons in antibonding orbitals) / 2. This gives an indication of the bond strength.
Frequently Asked Questions about the MO Diagram for O2
The MO diagram for O2 is a powerful tool for understanding the electronic structure and properties of the oxygen molecule. However, many questions arise regarding its construction, interpretation, and applications. This section aims to address some of the most commonly asked questions, clarifying misconceptions and reinforcing key concepts related to the MO diagram for O2. Addressing these frequently asked questions helps solidify a thorough understanding of the topic.
Why is the MO diagram for O2 important?
The MO diagram for O2 is crucial because it explains the molecule’s paramagnetism (due to unpaired electrons), its bond order (indicating bond strength), and its overall stability. This information is fundamental to understanding oxygen’s reactivity and its role in various chemical and biological processes. The diagram provides a quantitative framework for analyzing the molecule’s electronic structure, far exceeding simple Lewis structures in this context. It’s indispensable for accurate predictions of chemical behavior.
How does the MO diagram for O2 differ from a Lewis structure?
A Lewis structure provides a simplified representation of bonding, showing only the valence electrons involved in bonding. The MO diagram, however, gives a more comprehensive picture by detailing all the molecular orbitals (bonding and antibonding), their energies, and electron occupancy. This leads to a more accurate description of the electronic structure and properties, including bond order, magnetic properties, and detailed electron distribution. The MO diagram explicitly reveals the presence of unpaired electrons in O2, a feature not readily apparent in the Lewis structure.
What are the limitations of using the MO diagram for O2?
While highly valuable, the MO diagram for O2 has limitations. Simpler models often oversimplify the complex interactions between atomic orbitals. Accurate energy level predictions typically require computational methods, and approximations are frequently involved. Furthermore, the model doesn’t directly account for vibrational or rotational effects on the molecule’s overall energy. Despite these limitations, it remains an essential tool for understanding oxygen’s electronic structure.
Key Aspects of the MO Diagram for O2
Understanding the MO diagram for O2 hinges on grasping several key aspects. These aspects, when considered together, provide a comprehensive understanding of the molecule’s electronic configuration and consequential properties. Focusing on these individual elements allows for a stepwise comprehension of the overall structure and significance of the diagram.
Bond Order
The bond order, calculated from the MO diagram, quantifies the strength and stability of the oxygen-oxygen bond. A higher bond order implies a stronger and more stable bond. The calculation involves subtracting the number of electrons in antibonding orbitals from the number of electrons in bonding orbitals, and dividing the result by two. This provides a crucial measure of the chemical bond’s stability.
Paramagnetism
The presence of unpaired electrons in the *2p orbitals explains oxygen’s paramagnetism, a property where the molecule is attracted to magnetic fields. This is a direct consequence of the electron configuration revealed by the MO diagram. The unpaired electrons are essential for understanding oxygen’s behaviour in magnetic fields.
Orbital Energies
The relative energies of the molecular orbitals in the diagram affect the electron configuration and therefore the properties of the oxygen molecule. Accurate energy level calculations frequently employ computational techniques. These energies are critical in dictating electron distribution, affecting bond order and reactivity.
Electron Configuration
The electron configuration, determined by filling the molecular orbitals according to the Aufbau and Hund’s rules, defines the overall electronic structure of O2. This configuration dictates all aspects of its chemical behavior. It underpins the molecule’s physical and chemical properties.
These key aspects are interconnected; changes in one directly affect the others. For example, a change in orbital energies alters the electron configuration and consequently the bond order and magnetic properties. Understanding these interrelationships is vital for a complete grasp of the information contained in the MO diagram for O2.
The MO diagram for O2 provides a sophisticated picture beyond what simpler models can offer. The diagram facilitates a precise understanding of the electronic interactions within the molecule, leading to a deeper understanding of its properties and reactivity.
Tips for Understanding the MO Diagram for O2
Mastering the interpretation and construction of the oxygen molecule’s MO diagram requires a systematic approach. Understanding the underlying principles and employing helpful strategies improves comprehension significantly. This section highlights several practical approaches to enhance comprehension of this complex yet fundamental concept.
Start by reviewing the fundamental principles of molecular orbital theory. Understanding the concepts of bonding and antibonding orbitals, orbital overlap, and the filling of orbitals according to the Aufbau principle and Hund’s rule is crucial. A solid foundation in these principles is essential for effectively interpreting the diagram’s information.
Start with the Atomic Orbitals
Begin by visualizing the atomic orbitals of individual oxygen atoms before combining them. This helps in understanding the origin of each molecular orbital.
Focus on Symmetry
Symmetry considerations are critical in constructing the MO diagram. Pay attention to the symmetry of the atomic orbitals and how they combine to generate molecular orbitals of different symmetries.
Use Online Resources
Utilize online resources such as interactive MO diagram generators and tutorials. These tools provide visual aids and practice exercises.
Practice Drawing the Diagram
Practice drawing the MO diagram repeatedly. This reinforces your understanding of the energy levels and electron filling.
Relate the Diagram to Properties
Connect the features of the MO diagram (bond order, electron configuration) to the observable properties of O2 (paramagnetism, bond strength).
The MO diagram for O2 serves as a cornerstone in understanding molecular behavior. Its accurate construction and interpretation require a thorough understanding of molecular orbital theory and its underlying principles. By systematically working through the process, one can gain valuable insights into the electronic structure and properties of diatomic oxygen.
The MO diagram for O2 is not simply a diagram; it’s a representation of the intricate electronic dance within the oxygen molecule. Understanding its construction and interpretation unlocks a deeper understanding of oxygen’s reactivity, magnetic properties, and its pivotal role in countless chemical processes.
In conclusion, the MO diagram for O2 is a powerful tool for understanding molecular electronic structure and properties. Mastering its interpretation requires a solid grasp of molecular orbital theory and a systematic approach. The benefits gained extend far beyond a single molecule, impacting our understanding of chemical bonding and reactivity across a vast range of chemical systems.
Ultimately, a comprehensive understanding of the MO diagram for O2 provides a foundation for tackling more complex molecules and advanced concepts in chemistry.
Youtube Video: